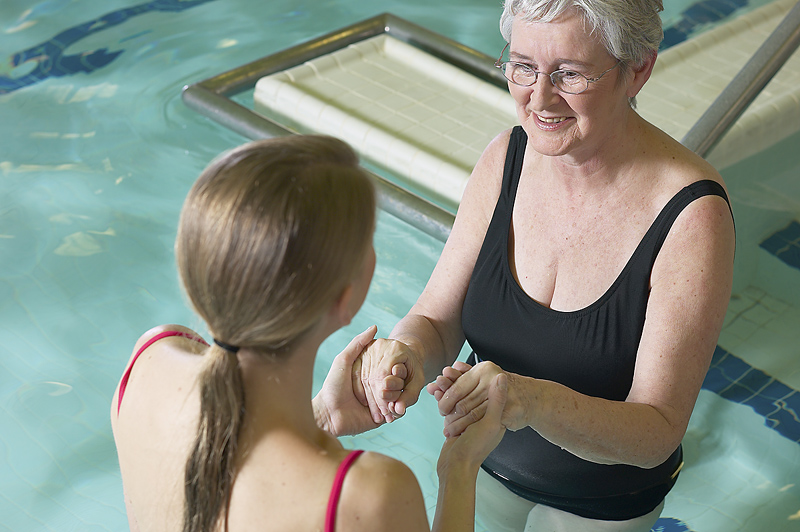
MONDAY, Oct. 11 (HealthDay News) — The first patient to be treated in a U.S.-government-approved study involving human embryonic stem cells has been injected with millions of the potentially life saving cells.
The patient, being cared for at the Shepherd Center in Atlanta, is partially paralyzed following a spinal cord injury. The center specializes in treating these types of injuries.
According to the trial’s protocol, patients must receive the stem cell injection within 14 days of the injury. The trial only involves patients with spinal cord injuries.
“We’ve known about this for a long time, we’ve been waiting for it to happen and we hope it goes well. Definitely it’s a step forward,” said Susan L. Solomon, CEO of the New York Stem Cell Foundation.
Paul Sanberg, professor of neurosurgery and director of the University of South Florida Center for Aging and Brain Repair in Tampa, added: “Clearly this bodes well, in the sense of getting stem cells to the clinic, especially in spinal cord injury. This is a safety study, and once that continues, hopefully there will be good efficacy.”
While many scientists and physicians are hailing the trial as a landmark, others have expressed some nervousness.
“There’s a lot of angst around these trials,” Evan Y. Snyder, director of the stem cell program at the Sanford-Burnham Medical Research Institute in San Diego, told the Washington Post. “There’s going to be this perception that if the cells do not perform well, the entire field will be illegitimate.”
Spinal cord injuries are just one of several conditions and diseases that scientists hope can one day be treated, cured or prevented with stem cell therapy. Others include Alzheimer’s disease, Parkinson’s and diabetes.
Although researchers around the world have made strides in the field, until now, no clinical trials have gotten under way in the United States.
The current Phase I trial, sponsored by Geron Corp. of Menlo Park, Calif., is mainly looking at the safety of using embryonic stem cells in this context. If all goes well, later trials will assess the strategy’s effectiveness.
In an announcement released Monday, Geron president and CEO Dr. Thomas B. Okarma said that “initiating [this] clinical trial is a milestone for the field of human embryonic stem cell-based therapies.”
According to the company statement, another center, Northwestern Medicine in Chicago, is also enrolling patients for a similar trial.
In all, seven sites will be involved in the study, the Post reported.
Embryonic stem cell therapy has been a major source of controversy and political drama for years. Early in President George W. Bush’s first term, his administration banned federal funding for research using newly created embryonic stem cells, citing ethical concerns that these cells represented viable human life.
That ban was overturned by the Obama administration, but in late August U.S. District Court Judge Royce Lamberth ruled that federal funding of embryonic stem cell research did violate a 1996 law prohibiting the use of taxpayer dollars for such work. The Obama administration appealed that decision.
Soon after, an appeals court issued a temporary suspension of the reinstituted ban until it could hear full arguments over the next few weeks.
In the wake of that stay, U.S. government officials announced that researchers at the National Institutes of Health would resume working with embryonic stem cells.
Meanwhile, results of a new Harris Interactive/HealthDay poll released last week found that a wide range of Americans, including Republicans, Catholics and born-again Christians, supported embryonic stem cell research. Almost three-quarters (72 percent) of the adults surveyed believe that scientists should be allowed to use embryonic stem cells left over from in vitro fertilization procedures to search for potential treatments or ways to prevent diseases such as Parkinson’s, Alzheimer’s and diabetes.
More information
Find out more about stem cell research at the U.S. National Institutes of Health.