FDA Upgrades Recall on 160,000+ Bottles of Thyroid Medication
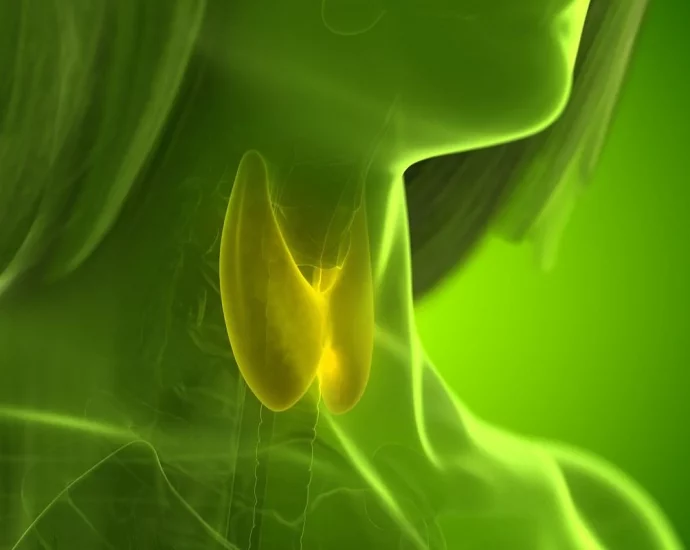
THURSDAY, July 24, 2025 (HealthDay News) — The U.S. Food and Drug Administration (FDA) has upgraded a recall of a commonly prescribed thyroid medication due to what it described as “subpotent” active ingredients. The recall of more than 160,000 bottles of levothyroxine sodium, which went into effect June 20, wasContinue Reading





-690x550.jpg)