SUNDAY, June 2 (HealthDay News) — A new drug called lambrolizumab appears to improve outcomes in patients with advanced melanoma, according to the results of a phase 1 trial.
Lambrolizumab is an antibody that works by revealing the cancer to the immune system so it can mount a response and kill the cancer cells with few serious side effects, the researchers said.
“This is early, but it’s very encouraging,” said lead researcher Dr. Antoni Ribas, a professor of medicine at the University of California, Los Angeles.
“This is a new class of drugs for cancer that are giving benefits in patients with melanoma, in terms of having a high rate of tumor responses that are durable in patients with metastatic melanoma,” he said.
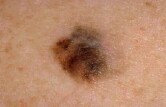
Melanoma is the deadliest type of skin cancer and, until recently, there was no effective treatment, Ribas said. In metastatic melanoma, the cancer has spread.
One of the ways some cancer cells fool the immune system is with a protein called PD-L1 on their surface, which renders the cancer invisible. “PD-L1 is a way the cancer tries to conceal itself or hide from the immune system,” Ribas said.
Lambrolizumab blocks the protein and “exposes the cancer to the immune system,” he said.
Ribas said that lambrolizumab might also be effective against other cancers, and has already been tested in patients with lung cancer.
The new study was a “phase 1b” trial, which seeks to determine if a drug is safe and also looks for signs of effectiveness. More testing and randomized trials are needed before the drug could become available, Ribas said.
The report was published online June 2 in the New England Journal of Medicine to coincide with the Sunday presentation of the findings at the annual meeting of the American Society of Clinical Oncology in Chicago.
For the trial, 135 patients with advanced metastatic melanoma were placed in three groups and treated with different regimens of lambrolizumab.
The researchers found that, overall, the drug improved cancer in 38 percent of the patients regardless of dose. Specifically, 25 percent of the patients who received the lowest dose showed improvement as did 52 percent of those given the highest dose.
In all, 77 percent of the patients showed some response to treatment, they noted.
How long the positive response lasts isn’t known. Five patients were taken off the drug because their cancer worsened. So far the longest response has been over one year, Ribas said.
Most side effects with lambrolizumab were mild and easily managed, he added. These included fatigue, fevers, skin rash, loss of skin color and muscle weakness.
According to Ribas, 13 percent of patients had more serious side effects, including inflammation of the lung or kidney, and thyroid problems.
The research was funded by Merck Sharp & Dohme, the maker of lambrolizumab.
In April, lambrolizumab received “breakthrough therapy” designation from the U.S. Food and Drug Administration, which means the agency will expedite reviewing the data to speed up getting the drug approved, Ribas said.
One dermatologist welcomed the news of the study results.
“I had a patient who was treated with this and it is definitely a lifesaver,” said Dr. Doris Day, a dermatologist at Lenox Hill Hospital, in New York City. Before being treated with lambrolizumab her patient had moved to California, but kept Day updated.
Current treatment for advanced melanoma is interferon, which has severe side effects, Day said. “You kind of wish you were dead, because you feel awful,” she explained. Lambrolizumab, however, has very mild side effects, she added.
“Life extension is one thing, but if you can’t extend life without quality then there’s really no point. My patient did well and had good quality of life,” Day said.
“Having metastatic melanoma may not be as much of a death sentence as it was,” Day noted. “There is hope. There are options now that extend life and quality of life.”
More information
To find out more about melanoma, visit the U.S. National Cancer Institute.