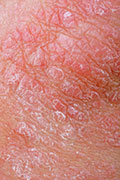
WEDNESDAY, July 9, 2014 (HealthDay News) — A new psoriasis drug delivered dramatic results in two clinical trials, perhaps heralding an effective new treatment for patients with the chronic skin disease.
The drug, secukinumab, was stacked up against an inactive placebo and one of the best psoriasis medications on the market.
“Over a quarter of patients have not a dot of psoriasis left,” said study co-author Dr. Mark Lebwohl, chairman of dermatology at the Icahn School of Medicine at Mount Sinai in New York City.
“Over half the patients have a 90 percent improvement in their psoriasis, and that means there’s hardly any psoriasis left. This kind of data is better than anything we’ve seen in the past,” he added.
The study results were published online July 9 in the New England Journal of Medicine.
Psoriasis causes overproduction of skin cells, which results in thick red marks and flaky white lesions that itch and burn. As many as 7.5 million Americans — about 2.2 percent of the population — have psoriasis, according to the National Psoriasis Foundation.
Secukinumab is a laboratory-engineered antibody that targets interleukin-17A, a pro-inflammatory protein in the body that has been previously linked to psoriasis.
The strong response to the new drug shows that researchers likely have singled out a primary cause of psoriasis, said Dr. Abby Van Voorhees, director of the Psoriasis and Phototherapy Center at the Hospital of the University of Pennsylvania.
The study “really does validate the type of cell that we think is sentinel in psoriasis,” said Van Voorhees, chair of the National Psoriasis Foundation’s medical board. The report also “advances our understanding of the disease, as well as gives us a new medication that will be very effective,” she said.
In one of the two trials, doctors randomly gave the injectable medication to two-thirds of 738 psoriasis patients. They received a dose of either 300 milligrams or 150 mg weekly for five weeks and then every four weeks thereafter. The remaining third received a placebo.
Within 12 weeks, four out of five patients who received 300 mg of secukinumab experienced a 75 percent improvement in their psoriasis symptoms, compared to one of every 20 patients who received a placebo, the study found.
About 59 percent of patients receiving the higher dose of secukinumab reported a 90 percent improvement in symptoms, and more than 28 percent said their psoriasis had cleared up completely.
“Not only was this phenomenally effective, but it was very quick,” Lebwohl said.
The other trial reported similar results. The study provided either 300 mg or 150 mg of secukinumab to half of a pool of 1,306 psoriasis patients, with doses delivered the same as in the first trial.
Another quarter received etanercept (Enbrel), which is one of the top treatments for psoriasis. The final quarter of patients received a placebo.
In this trial, more than three-quarters (77 percent) of patients receiving the high dose of secukinumab experienced a 75 percent improvement in their psoriasis symptoms within 12 weeks, compared with 44 percent of those who received Enbrel and about 5 percent who got a placebo, the investigators found.
About 54 percent of secukinumab patients reported a 90 percent improvement in their symptoms, compared with 20 percent for Enbrel, the study found. One out of four patients reported that their psoriasis had completely cleared up with secukinumab, compared with one out of 20 for Enbrel.
In both trials, secukinumab proved relatively safe. The only side effect appears to be an increased risk of infection, which was higher than placebo in both studies but similar to that of Enbrel.
“The psoriasis drugs that we have used in past years suppressed big parts of the immune system, and because of that they had a lot of side effects,” Lebwohl said. “This one appears to block the smallest part yet, and it appears to block the right part because it is the most effective of all the drugs to treat psoriasis yet.”
Van Voorhees said the trials add up to a “very promising new agent” for treating psoriasis. The drug now awaits U.S. Food and Drug Administration approval.
“It’s more effective than one of our gold-standard therapies, it’s well-tolerated, and the only risk seems to be an increased risk of infection,” Van Voorhees said.
The trials were funded by the drug’s manufacturer, Novartis Pharmaceuticals.
More information
For more on psoriasis, visit the U.S. National Library of Medicine.
Copyright © 2026 HealthDay. All rights reserved.

