FRIDAY, Nov. 21, 2014 (HealthDay News) — A clinical trial of hydroxyurea therapy for children with sickle cell anemia has been halted a year early because the results show it is a safe and effective way to manage the disease and reduce the risk of stroke.
The announcement about the research, which was conducted at 25 medical centers in the United States and Canada, was made this week by the U.S. National Heart, Lung, and Blood Institute (NHLBI).
Researchers compared monthly blood transfusions with daily hydroxyurea pills among children with sickle cell anemia who were at high risk of stroke. To determine this, they measured the velocity of blood flow to the brain in these young patients.
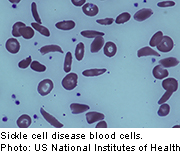
With sickle cell anemia, red blood cells become stiff and sickle-shaped, blocking blood flow throughout the body. Hydroxyurea was first developed as a cancer drug, but with sickle cell anemia it reduces the number of these abnormally shaped red blood cells, the researchers said.
Early results showed that hydroxyurea “works as well as blood transfusions which lowers the risk of the child having a stroke,” principal investigator Dr. Russell Ware, director of hematology at Cincinnati Children’s Hospital Medical Center, said in a center news release.
“This critical research finding opens the door to more treatment options for clinicians trying to prevent strokes in children living with the sickle cell disease,” NHLBI Director Dr. Gary Gibbons said in the news release.
The study began in September 2011 and enrolled 121 children, aged 4 to 16, with sickle cell anemia who showed an increased risk of stroke.
More information
The U.S. National Heart, Lung, and Blood Institute has more about sickle cell anemia.
Copyright © 2026 HealthDay. All rights reserved.