FRIDAY, Aug. 24 (HealthDay News) — An experimental drug is showing some promise in slowing the decline in thinking and memory that comes with Alzheimer’s disease, especially in people with milder forms of the illness.
Drug maker Eli Lilly & Co. announced Friday that solanezumab did not slow mental decline in each of two phase 3 clinical trials, but when the data from the two studies was combined a statistically significant and positive effect was seen. This effect seemed to be concentrated in patients with mild cases of Alzheimer’s, the company said.
“We recognize that the solanezumab studies did not meet their primary endpoints, but we are encouraged by the pooled data that appear to show a slowing of cognitive [thinking/memory] decline,” Lilly chairman, CEO and president John Lechleiter said in a company news release.
Because neither trial met its hoped-for main goal, U.S. Food and Drug Administration approval may still be a ways off, experts noted. But Lilly said it plans to continue to study the drug and, in the meantime, Lechleiter said the company “will discuss these data with regulatory authorities to gain their insights on potential next steps.”
In a statement, the Alzheimer’s Association said extending the trial is good news because “people who were in the phase 3 trials will have an opportunity to continue taking the drug. This will give us further insight into the effects of this drug over a longer period of time.”
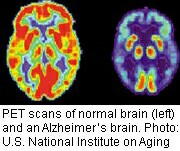
According to the Alzheimer’s Association, more than 5.4 million Americans are living with Alzheimer’s disease and that number is expected to rise. While certain medications can temporarily ease symptoms, there is no effective treatment that slows, stops or reverses the mental decline associated with Alzheimer’s disease.
Solanezumab is a monoclonal antibody, a naturally occurring human antibody that is genetically tweaked in a laboratory and then cloned in large numbers and introduced back into the patient to target disease. The drug targets amyloid beta protein plaques in the brain. These deposits have long been linked with Alzheimer’s disease, although it’s been unclear if they are merely associated with the disease or help to cause it.
The two phase 3 clinical trials of solanezumab lasted 18 months and included more than 2,050 patients with mild-to-moderate Alzheimer’s disease from 16 countries around the world. More results from the trials are expected to be released at two medical meetings to be held in October, Lilly said.
The Alzheimer’s Association called the solanezumab findings “encouraging.”
“If this finding can be duplicated, it suggests that an Alzheimer’s therapy targeting beta amyloid can have a beneficial effect on cognitive abilities in people with mild to moderate Alzheimer’s,” the group said in its statement. “That would be a major step forward in the fight against Alzheimer’s disease.”
One expert remained cautious, however.
“It’s a soft finding at this point in time,” said Dr. Ronald Petersen, a neurologist with Mayo Clinic in Rochester, Minn., and chair of the federal Advisory Council on Alzheimer’s Research, Care and Services. He said the effect seen in the trials was “a bit subtle, but it seems to be real and it may in fact mean that the drug is doing what it’s supposed to do.”
However, he added that “the conundrum in the field remains that treating patients with established dementia due to Alzheimer’s disease may be too late.” For many patients with Alzheimer’s disease, “we may be in fact trying to intervene after sufficient damage is done in the brain that we really cannot reverse it,” Petersen said.
More information
Find out more about Alzheimer’s disease at the U.S. National Institute on Aging.